
It’s a growing category that has become the fascination of many consumers. But are companies moving too fast to make and sell these products without established FDA regulations?
Products containing cannabidiol (CBD) seem to be everywhere, from corner stores to mail-order giants. Consumers can pick from supplements and sprays to balms and brownies as they seek the serenity promised by this cannabis derivative. Cannabis beverages are particularly appealing to consumers. According to a survey by High Yield Insights, 17% of current CBD respondents, led by men aged 21–34, reported consuming CBD beverages, and 30% of nonusers reported interest.1 With a projected average annual growth in the United States of 75%, from 2018 to 2023, the emerging category of CBD beverages is hard to ignore.
Overview of CBD
Of the Cannabis sativa plant, marijuana and hemp are the best-known strains; CBD and tetrahydrocannabinol (THC) are the best-known bioactive compounds. More than 80 phytocannabinoid compounds have been identified in the Cannabis sativa plant alone.2 CBD is the nonintoxicating cannabinoid associated with health benefits, including analgesic properties. THC, the intoxicating compound, features prominently in marijuana but is present at less than 0.3% in hemp; therefore, hemp is the source of choice for CBD.
A quick scan of the available research demonstrates broad interest in the functions and health-supporting roles of the body’s endocannabinoid system, including mental and physical health, as well as disease treatment and prevention. Plant-derived cannabinoids (ie, phytocannabinoids) have been researched as potential therapeutic options because of their modulation of the endocannabinoid system.2
In her FNCE® 2019 presentation, “CBD is Everywhere! Navigating the MNT and Its Role in the Marketplace,” Laura Lagano, MS, RDN, CDN, highlighted more than two dozen conditions CBD usage can help alleviate. She noted that CBD acts by impacting serotonin, opioid, dopamine, and other receptors located throughout the body.
Studies suggest the effects of CBD on the activity of the endocannabinoid system have the potential to benefit treatment for a broad range of conditions, including neurodegenerative, cardiovascular, and inflammatory disorders; obesity and metabolic syndrome; cachexia; chemotherapy-induced nausea and vomiting; and tissue injury and pain.3 Clinical trials, however, have focused primarily on THC and its targets, CB1 receptors. To date, CBD studies are limited to preclinical and pilot studies that elucidate its anti-inflammatory, antioxidative, and neuroprotective effects.4
One preliminary study published in 2011 compared the effects of simulated public speaking on healthy control patients, patients with diagnosed but untreated social anxiety who received a single 600-mg dose of CBD, and patients with diagnosed speaker anxiety who received a placebo.5 Subjective mood and self-deprecation tests and physiological tests were measured several times during the public speaking exercise. Patients who were pretreated with CBD showed significantly less anxiety, cognitive impairment, and discomfort during the simulation.
Janice Newell Bissex, MS, RDN, FAND, a holistic cannabis practitioner, culinary nutritionist, and owner of Jannabis Wellness in Melrose, Massachusetts, says, “We are beginning to learn about the variety of health benefits of CBD to help alleviate pain, anxiety, insomnia, muscle spasms, inflammation, depression, and possibly autism. It isn’t a cure-all, but it can improve or relieve a lot of conditions in a more natural way. Some of my clients have been able to decrease their use of antianxiety and pain medications after adding cannabis to their regimen.”
Questions remain, however, regarding the safety of CBD, including the effects of long-term use and using multiple CBD-containing products simultaneously. CBD use has been associated with liver toxicity, extreme sleepiness, interaction with certain medications, and potential harm to a fetus or breast-fed baby. More research is needed to better understand the benefits and drawbacks of CBD and to increase knowledge about the endocannabinoid system, its potential modulators, and its impact on various conditions.
Regulatory Status in Flux
The 2018 Farm Bill removed hemp from the list of Schedule 1 narcotics (where it previously was categorized with marijuana) and legalized its cultivation, opening the door for production of topical and other products containing CBD. The Farm Bill retains FDA’s authority to regulate the sale and marketing of CBD-containing foods. However, under the federal Food, Drug, and Cosmetic (FD&C) Act, because CBD is an active pharmaceutical ingredient in an approved drug, Epidiolex, food (or beverage) products containing CBD can’t be introduced into interstate commerce, that is, manufactured in one state and sold in another, according to the FDA.6
The FDA issued warning letters in late November 2019 to companies regarding CBD in their food and beverage products and for higher amounts of THC than are stated on labels or legally permitted. In a November 26, 2019, consumer notice, the FDA stated that “under the FD&C Act, it’s illegal to introduce into interstate commerce any human or animal food to which certain drug ingredients, such as CBD, have been added. In addition, the FDA isn’t aware of any basis to conclude that CBD is GRAS [generally recognized as safe] among qualified experts for its use in human or animal food. There also is no food additive regulation which authorizes the use of CBD as an ingredient in human food or animal food, and the agency is not aware of any other exemption from the food additive definition that would apply to CBD. CBD is therefore an unapproved food additive and its use in human or animal food violates the FD&C Act for reasons that are independent of its status as a drug ingredient.”7 Individual states have passed legislation allowing the sale of CBD products.
Many companies, particularly larger manufacturers, have avoided entering the market until FDA regulations are established. Moving forward, FDA regulation of CBD in beverages would provide rules and guidelines for manufacturers and could protect consumers from substandard or inaccurately labeled products.
Regulatory inconsistencies also confuse consumers. In October 2019, the Grocery Manufacturers Association reported on research regarding consumers’ assumptions about CBD, stating that “a whopping 92% of American consumers incorrectly assume or have no idea if CBD is federally regulated. Upon learning that CBD products are not regulated federally, 82% of respondents were concerned.”8
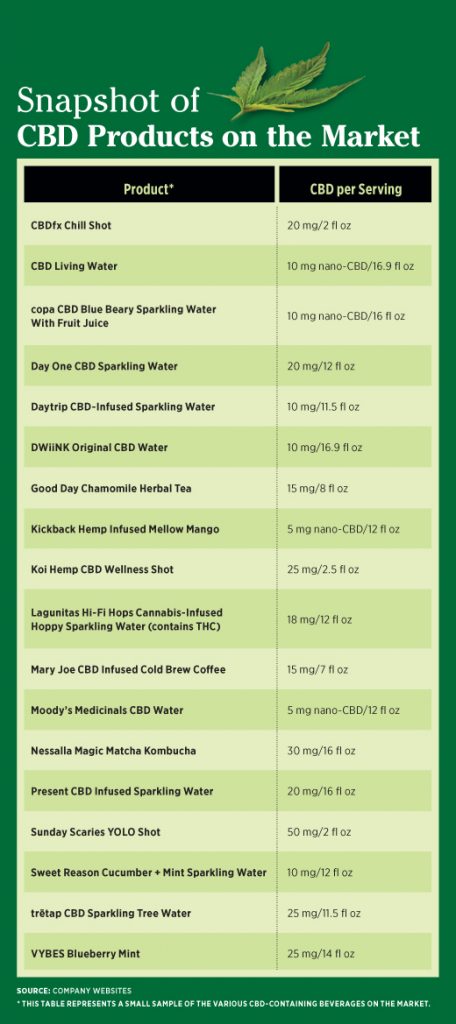
CBD Beverage Marketplace
Despite the fact CBD isn’t federally regulated, beverages are a hot bed of innovation regarding CBD. According to Innova Market Insights, beverages hold seven of the top 10 positions for new product launches containing CBD—flavored bottled water, tea, carbonates, noncarbonated soft drinks, iced tea, ready-to-drink sports drinks, and unflavored bottled water. CBD-infused beverages are more popular than beverages containing THC or CBD plus alcohol. Despite the lack of clarity in regulations, CBD is widely available, easy to add to beverages, and less strictly regulated than THC.9 CBD beverages appeal to general consumers for their therapeutic benefits and calming properties and to those who use it medicinally to treat a variety of conditions. Because CBD doesn’t have the intoxicating properties that THC does, it’s less likely to have negative effects if overconsumed.1
“Incorporating CBD into beverages is relatively easy using a CBD powder or CBD dissolved in a water-soluble liquid,” Bissex says. “Most research studies use a CBD isolate product so the dose can be precisely controlled, and many manufacturers add this inexpensive CBD isolate to their products. The downside is that because the isolate doesn’t have the synergistic benefit of the other cannabinoids—the flavonoids and terpenes found in broad or full spectrum CBD products—you need a much higher dose for efficacy.”
Bissex notes that hemp was a legal pharmacological ingredient until less than 100 years ago. “Then it became vilified because of its association with THC and the overconsumption of THC. Although the hemp plant is extremely low in THC, consumption could trigger a positive drug test. That is why it’s important to buy CBD products that have zero THC. The hemp plant has hundreds of beneficial phytocompounds, so look for CBD sources with just the THC removed rather than CBD isolates that have only CBD.”
Broad spectrum CBD includes all of the cannabinoids and terpenes from the cannabis plant except THC, while full spectrum also includes THC. The term “entourage effect” describes a theory that a product with a broad or full spectrum of terpenes, flavonoids, cannabinoids, and other botanicals delivers synergistic ingredients that can enhance one another’s efficacy. Beverages also may incorporate other functional compounds such as L-theanine, an amino acid analogue said to promote relaxation.
One factor to note is that CBD doesn’t mix well with water and can separate out or adhere to the inside of the beverage bottle over time, so it’s hard to know exactly how much gets consumed. Separation also decreases bioavailability. In addition, one manufacturer states that 90% or more of the CBD particles in a typical CBD beverage product are too large to be incorporated into cells in the body.10 Some manufacturers try to overcome this by using nano-emulsion technology to improve bioavailability. In nano-enhanced CBD, also called nano-CBD, clustered CBD isolate molecules are broken down into extremely small, water-soluble particles that are said to be absorbed rapidly and able to cross the blood-brain barrier.
The CBD dosage in beverages currently on the market generally ranges from 10 mg to about 25 mg per serving. “It’s important to ask clients how much they’re drinking,” Bissex says. “Someone who is having several drinks a day may be getting much more than the minimally effective dose of CBD.” The form of CBD, namely, conventional vs nano-enhanced, impacts the speed of absorption and action.
Cautionary Notes
Due to questions surrounding federal regulation, the complexities of manufacturing, and the sourcing of CBD, “the CBD beverage landscape is confusing and inconsistent,” Lagano says. “CBD and cannabis use in beverages is not standardized, and the sources of CBD vary. Some companies try to circumvent FDA guidelines by claiming their products contain hemp oil. But if that oil is from hemp seeds, it will contain little CBD. The source needs to be hemp/CBD oil. Bioavailability is variable, and extraction methods vary. Effects will differ based on the form and format of the CBD, along with a person’s biochemical individuality, microbiome, and endocannabinoid system. The good news is that the industry is trying to mimic the supplement industry with detailed labels.”
In September 2019, the Academy of Nutrition and Dietetics (the Academy) issued a public policy statement that recommends caution around consuming food items with CBD because of the “lack of rigorous scientific trials and data regarding dosing, effectiveness, and safety.”11 The Academy also noted its intention to support advocacy efforts to secure funding to close the research gap regarding CBD and THC as food additives, including research on differential effects across the lifecycle; monitoring legislative and regulatory initiatives related to CBD and marijuana as the evidence base is further established, and reviewing and updating policy stances and recommendations accordingly; and communicating the potential for misuse, consumer confusion, and a lack of sufficient scientific substantiation in the marketing of CBD in food products and the use of associated health claims.
In the absence of regulations, product quality and CBD-related claims are inconsistent. Bissex highlights an FDA finding that a majority of CBD products on the market are mislabeled, including that 1 in 10 contain zero CBD. Lagano’s presentation at FNCE® emphasized the importance of third-party laboratory testing, labeling, quality seals, and Certified Good Manufacturing Practices (CGMP) to identify quality products with a reliable CBD content.
In the article “Clinicians’ Guide to Cannabidiol and Hemp Oils,” published in Mayo Clinic Proceedings, VanDolah and colleagues present the following questions pertaining to product quality that consumers should consider or discuss with a health professional before consuming any CBD product2:
• Does it meet the FDA’s CGMP standards, or European Union, Australian, or Canadian organic certification, and National Science Foundation international certification?
• Does the company have an independent adverse event reporting program?
• Is the product certified organic or eco-farmed?
• Have the company’s products been laboratory tested by batch to confirm THC levels <0.3% and no pesticides or heavy metals?
“I recommend only choosing products made from organically grown ingredients,” Bissex says. “Hemp and cannabis are bioaccumulators that gather chemicals, including toxins and pollutants, from the soil through their extensive root network.” Of interest, Bissex shares that hemp was planted after the Chernobyl nuclear disaster to decontaminate radioactive soil.
The CBD beverage industry is likely to grow and expand into more subcategories, including sports and recovery beverages and alcohol-CBD combinations. Dietitians who counsel clients on the use of CBD beverages can expect more transparency from companies regarding CBD source and content, guidance on dosing, information on bioavailability, and an expanded marketplace once the FDA establishes regulations.12 For now, inconsistencies in product quality, including declarations of CBD content, add to the challenge of guiding consumers toward responsible choices.
— Mindy Hermann, MBA, RDN, is a food and nutrition communicator based in metropolitan New York.
References
1. Giandelone E. Cheers to growth: the CBD beverage opportunity. High Yield Insights website. https://www.highyieldinsights.com/insights/2019/8/06/cbdbeverageopportunity. Published August 6, 2019.
2. VanDolah HJ, Bauer BA, Mauck KF. Clinicians’ guide to cannabidiol and hemp oils. Mayo Clin Proc. 2019;94(9):1840-1851.
3. Pacher P, Kunos G. Modulating the endocannabinoid system in human health and disease—successes and failures. FEBS J. 2013;280(9):1918-1943.
4. Zuardi AW. Cannabidiol: from an inactive cannabinoid to a drug with wide spectrum of action. Braz J Psychiatry. 2008;30(3):271-280.
5. Bergamaschi MM, Queiroz RH, Chagas MH, et al. Cannabidiol reduces the anxiety induced by simulated public speaking in treatment-naïve social phobia patients. Neuropsychopharmacology. 2011;36(6):1219-1226.
6. FDA regulation of cannabis and cannabis-derived products, including cannabidiol (CBD). US Food and Drug Administration website. https://www.fda.gov/news-events/public-health-focus/fda-regulation-cannabis-and-cannabis-derived-products-including-cannabidiol-CBD. Updated December 31, 2019.
7. What you need to know (and what we’re working to find out) about products containing cannabis or cannabis-derived compounds, including CBD. US Food and Drug Administration website. https://www.fda.gov/consumers/consumer-updates/what-you-need-know-and-what-were-working-find-out-about-products-containing-cannabis-or-cannabis. Updated November 25, 2019.
8. Federal regulation must catch up with unprecedented CBD boom. Grocery Manufacturers Association website. https://www.gmaonline.org/blog/federal-regulation-must-catch-up-with-unprecedented-cbd-boom/. Published October 28, 2019.
9. CBD-infused drinks tries to offer a more health conscious way to consume cannabis. Cision PR Newswire website. https://www.prnewswire.com/news-releases/cbd-infused-drinks-tries-to-offer-a-more-health-conscious-way-to-consume-cannabis-300904096.html. Published August 20, 2019.
10. What is nano-amplified CBD? Isodiol website. https://isodiol.com/isodiol-news/cbd-isolate/
11. Academy announces stance on CBD in food and beverage products. Academy of Nutrition and Dietetics website. https://www.eatrightpro.org/news-center/on-the-pulse-of-public-policy/from-the-hill/academy-announces-stance-on-cbd-in-food-and-beverage-products. Published September 24, 2019.
12. Jones K. Visualizing the boom in the CBD beverage market. Visual Capitalist website. https://www.visualcapitalist.com/boom-in-the-cbd-beverage-market/. Published August 1, 2019.
